The different types of cleaning agents used in housekeeping and how housekeepers should use them in houses and hotels. Share on X There are 4 different types of cleaning agents commonly used by housekeepers in private houses and hotels. Each of the cleaning agents has a specific purpose. And it is important to use them as intended. Otherwise dangerous and costly mishaps can occur.
The four types of cleaning agents used in housekeeping are:
- Detergents
- Degreasers
- Abrasives
- Acids
Training for correctly using these chemical cleaning agents is part of Polo & Tweed’s Housekeeping Course. To ensure housekeepers are knowledgeable in safety procedures. And best practices for using each type of cleaning agent.
In this article, we’ll cover the following aspects of our housekeeping job training. And using cleaning agents.
- Safety aspects of handling cleaning agents
- Labeling of chemical cleaning agents
- Chemicals used in different types of cleaning agents
- Appropriate use of the different types of cleaning agents
- Tips for using chemical cleaning agents
Safely Storing and Handling Cleaning Agents
Many of the chemicals used in cleaning agents can be harmful to human health and the environment. The chemicals are classified as irritants and/or allergenic fragrances. So there are strict regulations governing the concentration of these toxic chemicals in the cleaning agents.
However, if you are using and storing cleaning agents in an institution or a house. You’re obligated to follow the manufacturers’ instructions. Do this to ensure your own safety and use safe systems at work.
- Reading product labels carefully before using them.
- Avoid direct skin contact to prevent dermatitis and other skin irritations.
Labelling Definitions
Symbols or pictograms on the labels of cleaning agents indicate whether chemical ingredients are oxidising. Or highly or extremely flammable, toxic, harmful, irritant, corrosive. Or dangerous for the environment.
In the past, you could see many different symbols for a variety of definitions. However, the European Union recently phased in new labelling requirements for clarification which started in 2017. The old pictograms had orange backgrounds. The new pictograms have white backgrounds.
These two new symbols were added:

Indicates health hazards such as skin irritation or sensitisation. Or serious eye irritation. Or that a product could be harmful if swallowed.

Contains chemicals that pose serious health hazards including carcinogens. Or respiratory sensitizers, reproductive toxicity, target organ toxicity. Or even germ cell mutagens.
Safe Use Icons
The following icons and phrases can also be found on the packaging of cleaning agents. Depending on the space available on the label, only the icon may be displayed. So housekeepers should know what the icons mean and follow the recommendations.
![]() Keep away from children
Keep away from children
![]() Keep away from eyes. If product gets into eyes rinse thoroughly with water.
Keep away from eyes. If product gets into eyes rinse thoroughly with water.
 Sensitive or damaged skin.
Sensitive or damaged skin.
 Ventilate the room after use
Ventilate the room after use
 Do not change the container to store contents
Do not change the container to store contents
 Do not ingest. If you ingest the product then seek medical advice.
Do not ingest. If you ingest the product then seek medical advice.
 Do not mix with other products.
Do not mix with other products.
Detergents
Detergents are substances that contain soaps and/or surfactants (any organic substance/mixture). Use them for washing or cleaning jobs for the household, institutional or industrial purposes, including:
- Dishwashing
- Handwashing
- Laundry washing
- Fabric softeners
- All-purpose cleaners
- Bleaching
There are many cleaning products that contain detergents and they come in various forms. Including powders, tablets, concentrated liquids, liquid capsules, pastes or cakes.
Phosphorus in Detergents
You will commonly see phosphorus as an ingredient in detergents. However, it is a toxic chemical. There are strict limits on the amounts of phosphorus in detergents.
Phosphorus limit in laundry detergents
According to EU Detergent regulations, consumer laundry detergents (for use by non–professionals, including in public laundrettes) must not contain more than 0.5 grams of phosphorus. This is in the recommended quantity of the detergent used in a standard washing machine load. This applies to the recommended quality of detergent.
Phosphorus limit in dishwasher detergents
The total content of phosphorus allowed in a standard dosage expressed in grams or ml or number of tablets for the main washing cycle. This is for normally soiled tableware in a fully loaded dishwasher (12 place settings. It must not exceed 0.3 grams. However, there are provisions for water hardness.
Restrictions on Chemical Fragrances in Detergents
There are 26 chemical fragrances commonly used in detergents which are most often linked to causing allergic reactions. For this reason, the concentration of these fragrances must not exceed 0.01% by weight in detergents.
The Danger to Children of Liquid Laundry Detergent Capsules
Housekeepers working in private houses or establishments where children are present must avoid the risk of children getting hold of liquid laundry detergent capsules. And avoid putting them in their mouths.
Liquid laundry detergent capsules intended for single-use contain highly concentrated liquid detergent-insoluble packages. They normally have bright colours. Making them attractive to children. Who could mistake them for sweets!
Incidents of Poisoning
Poison Centres in several EU countries have reported a significant number of severe incidents of ingestion and eye damage involving infants and young children regarding liquid laundry detergent capsules. A higher accident rate compared to laundry detergents in other types of packaging.
The main symptoms and consequences of exposure to concentrated laundry detergent can be the following.
- If ingested: severe vomiting, coughing, respiratory disorders, nausea, drowsiness, and rash.
- In case of contact with the eyes: conjunctivitis, eye pain, eye irritation.
- In case of contact with the skin: skin rash, skin irritation, chemical burn.
Degreasers
You can use degreasers for heavy-duty work to remove grease, grime, dirt and oil from hard surfaces. Commercial kitchens generally use them. To remove grease from grills, ovens, and other metal surfaces. As well as from heavily soiled floors.
How Degreasers Work
Grease, oils and fats are organic dirt. Alkaline solutions and solvents break this down. Heavy-duty degreasers such as oven cleaners have a high pH (more alkaline). All-purpose cleaners for light dirt and dust have neutral pH.
Cleaning and degreasing agents on the pH scale
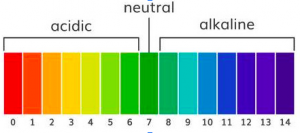
- All-purpose cleaners pH 6-8 (neutral)
- Cleaner-degreasers pH 9-10 (alkaline)
- Heavy-duty degreasers pH 11-13 (high alkaline)
- Oven cleaners pH 14 (extremely alkaline)
Common Ingredients in Degreasers
- Sodium Carbonate (soap ash)
- Sodium Meta Silicate
- Ethylenediaminetetraacetate (EDTA)
- Sodium Tripolyphosphate
- Kerosene
- Methylated spirits / white spirit
- Xylene
Safety Awareness When Using Degreasers
As degreasers are high-alkaline cleaning agents they can be corrosive. So housekeepers must closely follow the instructions on the product labels to ensure they are only used for their intended purpose. And to avoid damage to surfaces.
Health Risks
High-alkaline cleaners and degreasers can cause chemical burns to the skin. Use degreasers in well-ventilated areas along with skin and eye protection.
Some degreasing products contain ammonia or lye. Never mix this with bleach. Because it results in a chemical reaction, which will produce poisonous chlorine gas.
Safer Alternatives
You can use a variety of naturally alkaline ingredients in degreasers in place of strong chemicals to reduce health risks. Environmentally-friendly, non-toxic and non-fuming degreasers are now more popular in commercial kitchens. To prevent chemical contamination.
Abrasives
Abrasives are either powders or liquids you can use to wear off dirt from hard surfaces. Think of things such as sinks, floors, kitchen, and bathroom surfaces.
How Abrasives Work
The main ingredients in abrasives are usually small particles or minerals. The effectiveness of an abrasive agent depends on the coarseness of those particles. Their abrasiveness depends on the coarseness of the particles in the product.
For example, you can find fine grade of chalk (gilder’s whiting)in silver polish. So that it doesn’t scratch the silverware. Below are some of the other substances you can find in abrasive cleaners.
- Aluminum oxide
- Calcium carbonate
- Calcite
- Feldspar
- Quartz
- Silica
- Whiting (powdered chalk)
Caution when using abrasives
Housekeepers should always read the labels on abrasive cleaning agents. To make sure they use the appropriate product for the cleaning task at hand.
Coarse abrasives can damage surfaces such as plastic, fiberglass, glass, non-stick cookware. As well as painted woodwork, plated metal, and highly polished metal.
Housekeepers should also exercise caution when repeatedly using abrasive cleaners on hard surfaces such as sinks, bathtubs, and kitchen appliances. Because the abrasive agent gradually scratches the finish of these items.
The scratches become deeper over time and dirt becomes more deeply embedded. Requiring even stronger abrasives to clean out embedded dirtier and stains over time.
Abrasives and Disinfectants
Some abrasive cleaners also contain chemical or organic disinfectants. This is to kill bacteria at the same time. These disinfecting agents can include the following. Pine oil, quaternary ammonium compounds, or sodium hypochlorite (household bleach).
Chemical antimicrobial agents are regulated. So the product label states “disinfectant”. So make sure to closely follow the directions on the label.
Housekeeping Tips for Using Coarse Abrasives
- Always test abrasive cleaners on a small, inconspicuous area of the surface you are cleaning. Before you use the cleaner on the entire surface.
- Use sparingly and rub gently. Do
- Do not use abrasives on marble or other natural stone surfaces.
- Only use on surfaces not harmed by mild abrasives or acids
- Don’t allow abrasives to dry out on the surface you are cleaning
Acid Cleaning Agents
Acid cleaning agents are often highly concentrated solutions. Use them for the toughest cleaning jobs to dissolve mineral deposits (descaling) and ingrained grime.
Acid cleaners can be dangerous and highly corrosive. Make sure to handle them with extreme care. And dilute them according to the manufacturer’s instructions.
Uses for Acid Cleaning Agents
- Descaling mineral deposits
- Rust removal
- Tough stain removal
- Dissolving
- Cleaning masonry
- Mould removal
- Bathroom tile cleaner
- Restoring tarnished or discoloured metal
Acid Strengths
Different cleaning jobs with acid cleaners require different strengths or dilutions. Housekeepers often use acid cleaners to clean bathrooms and for dishwashers.
Very Mild Acid Cleaners
Vinegar and lemon juice are mildly acidic (about 5%) and have the benefit of being organic. Use them to remove hard-water deposits from glassware.
Very Strong Acidic Cleaners
Oxalic acid, Hydrochloric and sulfuric acid are strong acids. Use them as rust removers and toilet bowl cleaners. Note: they are very poisonous.
Safety Precautions When Using Acid Cleaners
Acid cleaning agents are highly toxic so housekeepers must follow label instructions exactly.
- Do not mix acid cleaning agents with other cleaners.
- Avoid contact with skin or your eyes
- Avoid splashing or spilling Acid cleaners on other materials
- Ventilate rooms when using acid cleaners
Tips for Using Different Types of Cleaning Agents
Apart from the daily routine of dusting and cleaning. Housekeepers face a cleaning job that requires special treatment.
To unblock a drain in the bathroom clogged with hair, soap, and toothpaste, for example. That requires one type of treatment. While a kitchen drain may have become clogged up with fat and grease. Which requires a different kind of cleaning agent.
Or perhaps you need to get the grime off a collection of glassware that hasn’t been touched for ages. Here are some guidelines on speciality cleaners for those special jobs:
| Cleaning Job | Specialty Cleaning Agent |
| Fabric stained with fungi, mould and mildew | Diluted liquid household bleach (sodium hypochlorite) |
| Kitchen drain clogged with fat and grease | Sodium hydroxide |
| Bathroom drain clogged with hair and soap | Sodium hypochlorite and sodium hydroxide |
| Glass stained with body oils | Solvents and alkaline cleaning agents |
| Glass stained with mineral salts | Acetic acid (vinegar) |
| Showerhead clogged with mineral deposits from hard water (limescale and rust) | Citric, oxalic, sulfamic or hydroxyacetic acid to dissolve the minerals |
| Tarnished metal | Kaopolite (clay) or fine hydrous silica |
Polo & Tweed Housekeeping Training Courses
Polo & Tweed’s housekeeping courses instill an eye for detail and a passion for ‘keeping house’. Housekeeping trainees perfect all the skills for professional housekeeping in institutions, private houses and luxury hotels.
Our housekeeping courses include group courses and bespoke, tailor-made courses for individuals that literally open up a world of opportunities with improved confidence, organizational and housekeeping management skills.
Housekeeping trainees benefit from personal guidance by highly experienced housekeeping professionals and practical hands-on experience to gain in-depth knowledge of housekeeping secrets for success.
Click here for more information about our housekeeping courses or call us with any questions at 0203 858 0233.

